
A Royal Reception for Obesity Research
Friday brought a perfect occasion to step back and appreciate a decade of progress in obesity research. Her Royal Highness the Crown Princess Mary of Denmark visited the obesity research center of Novo Nordisk in Seattle. Four years ago, that research center did not even exist.
Today, it’s generating an impressive stream of new drugs. And it’s promising to revolutionize obesity care by 2030.
Dim Prospects in 2008
Ten years ago, Sanofi walked away from an ambitious obesity research program. That decision came after the company withdrew its lead compound – rimonabant – from the market worldwide. In the U.S., it never even gained approval.
At the 2010 Cleveland Clinic Innovation Summit on Obesity, Sanofi CEO Chris Viehbacher explained the decision:
As long as we’re so worried about obesity being a lifestyle choice — that anyone can choose to be fat or thin — then I don’t think we’re going to have an ability to develop drugs. I don’t think right now we have a regulatory environment, a risk/benefit environment that would allow me as a CEO to take the risk of developing a drug for obesity.
At the same time, other companies, such as Merck, made similar decisions.
Looking at Obesity Through a Different Lens
Novo Nordisk was a company that competed with Sanofi in diabetes care. But it took a very different view of obesity. Sanofi looked at obesity as a condition with a simple solution. Weight loss. And as a matter of fact, most everyone else looked at it like that.
Fortunately, Novo Nordisk brought a view of obesity as a complex chronic disease. The company saw obesity overlapping with its core commitment to diabetes care. While others were walking away from obesity, Novo Nordisk stepped up. The company invested in obesity research.
First, it worked to define a role for its GLP-1 agonist, liraglutide, to treat obesity. Liraglutide was already becoming a blockbuster treatment for type 2 diabetes. So the company invested to show that at a higher dose, it could be an effective long-term treatment for obesity.
The key is looking at obesity as a complex, chronic metabolic disease. It’s not just a simple problem of excess weight to be lost.
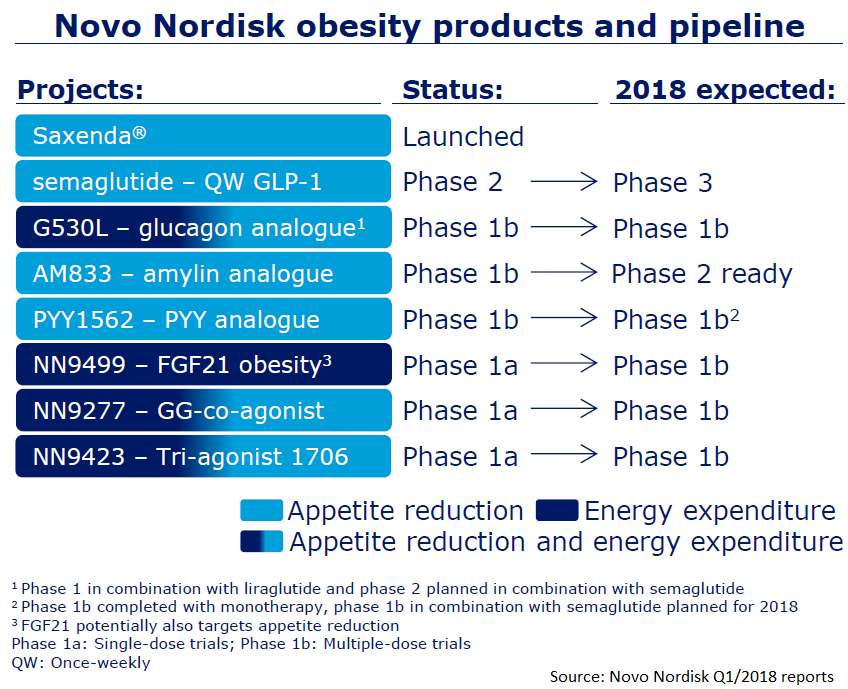
A Robust Pipeline
So now, Novo Nordisk has the most successful obesity treatment in the market – its Saxenda brand of liraglutide. But it also has a pipeline of seven other drugs in clinical development. Right now, a new GLP-1 agonist, semaglutide, is moving into phase 3 studies for obesity.
Also, AM833 is moving into phase 2 studies. This drug is an amylin analogue. If it succeeds, it might be the first of its class to be approved for treating obesity.
Raising the Bar for Obesity Care
The company aims to bring the kind of effectiveness seen with bariatric surgery into the realm of anti-obesity medicines. It’s a lofty goal. But we hope it succeeds and attracts competitors that will raise the bar even further.
If so, patients living with obesity will inevitably benefit from having better options.
Click here and here for more on the Crown Princess Mary’s visit to Seattle. For more on the potential role of amylin analogues in obesity treatment, click here.
Her Royal Highness the Crown Princess Mary of Denmark, photograph © Ted Kyle
Subscribe by email to follow the accumulating evidence and observations that shape our view of health, obesity, and policy.
May 5, 2018

May 05, 2018 at 3:26 pm, Allen Browne said:
I wish I knew someone at Novo-Nordisk to talk to about children with obesity. They are making remarkable progress to provide tools other than surgery for people with obesity. But the current paradigm for pediatric drug development is way too slow.
May 06, 2018 at 4:32 am, Ted said:
Granting that this process is slow, nonetheless it looks like they are sponsoring research to address some of your concerns, Allen. https://www.ncbi.nlm.nih.gov/pubmed/27979579