
Lilly Decides to Advance Tirzepatide for Obesity
It was odd. Lilly had a promising new drug for diabetes that delivered unusually good weight outcomes. But they weren’t sure about developing it for obesity. That was last October. Now, though, they seem to have decided to move ahead with their dual GIP/GLP-1 agonist, tirzepatide, for obesity. Pivotal phase 3 trials will begin this year.
This news went almost unnoticed in the flood of new studies presented at the American Diabetes Association’s scientific meeting this week.
Four New Presentations
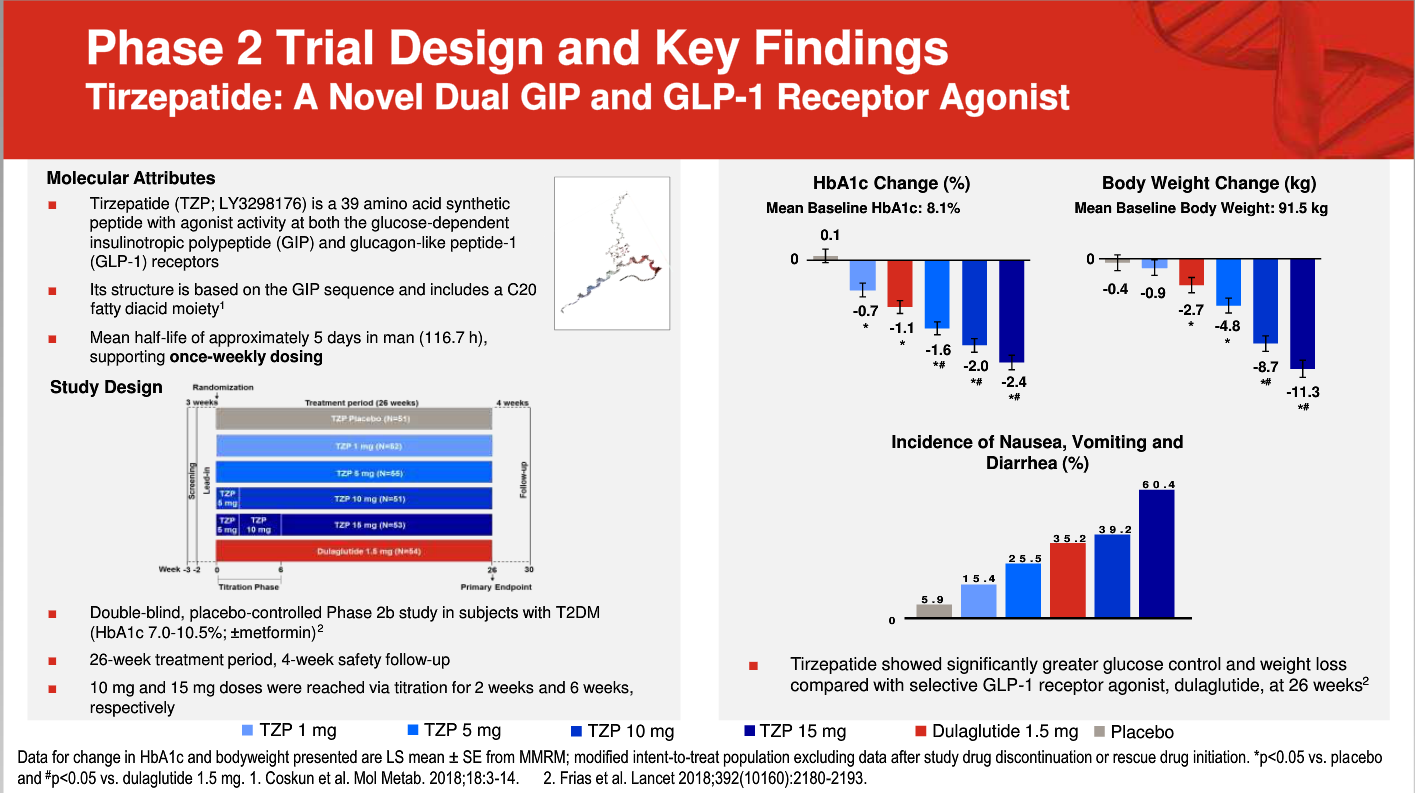
Lilly presented four new studies that are giving the company confidence to advance tirzepatide into phase 3 studies. In one, they dug deeply into the markers of efficacy from the phase 2 study of type 2 diabetes. Effects on beta cells and insulin sensitivity point to a unique clinical profile for this dual agonist.
Another study gave additional evidence of effects for obesity. Yet another provided insight on dosing regimens for better tolerability. And then finally, Lilly presented data on potential effectiveness in NASH. In sum, these early data are encouraging, though phase 3 studies will paint a much clearer picture.
The Daunting Challenge of Obesity
Questions remain about the tolerability of tirzepatide. GI side effects, especially nausea, were common in the early studies at higher doses. Lilly says they have worked out dosing regimens that will improve on this. We hope so. But the real story will come from the pivotal trials starting this year.
Obesity is a daunting challenge. We have more options now, but two of the four new drugs on the market (Qsymia and Contrave) have struggled for a lack of commercial success. Eisai has done a bit better with Belviq, but it’s no blockbuster yet. The company is seeking new labeling for cardiovascular safety from FDA that might help.
Only Novo Nordisk, with Saxenda, has found major success. Perhaps part of that success comes from looking at obesity as a chronic disease strongly linked to diabetes. Less successful competitors took a view that focused more on short-term weight loss. Not a good idea.
Lilly – with a strong history in diabetes – might bring strong competition to Novo Nordisk. Of course, that all depends on the clinical results they get in the pivotal studies for tirzepatide. Regardless, we’re glad to see them take up the challenge.
Click here for more from Lilly on their presentations at the ADA meeting. For their study of tirzepatide efficacy markers, click here for the abstract and here for the detailed poster. Finally, you can get slides with additional insights on tirzepatide here.
Duality, photograph © Elizabeth Haslam / flickr
Subscribe by email to follow the accumulating evidence and observations that shape our view of health, obesity, and policy.
July 14, 2019

June 20, 2019 at 1:07 pm, Donna Ryan said:
Ted, Where did you hear that Lilly was taking tirzepatide forward in obesity? I think they should, of course, but I haven’t been able to find something official Thanks, and all my best,
Donna
June 20, 2019 at 4:34 pm, Ted said:
In these slides from Lilly, Donna, you’ll find statements that they’re beginning phase 3 trials for obesity this year. They also discussed it in their investor briefing, which you can read here.